Nuclear Fission
A Little History
In 1932, English physicist and Nobel laureate James Chadwick discovered the neutron. A few years later, Enrico Fermi and his collaborators in Rome discovered that, if various elements are bombarded by neutrons then new radioactive elements are produced. Fermi had predicted that the neutron, being uncharged, would be a useful nuclear projectile, because it is uncharged and therefore receives no electric forces from the nucleus when it approaches the nuclear surface.
In 1939, German chemists Otto Hahn and Fritz Strassman, bombarded solutions of uranium salts with neutrons. They found by chemical analysis that, afterwards, a number of new radioactive elements were present. Repeated tests convinced them that barium was produced! This puzzle was solved within a few weeks by Lise Meitner and her nephew Otto Frisch. They showed that a uranium nucleus, having absorbed a neutron, could split, with the release of energy, into two roughly equal parts, one of which might well be barium. Frisch named the process fission.
The Physics behind Nuclear Fission
In a typical Uranium-235 fission event, a U-235 nucleus absorbs a thermal neutron, producing a compound nucleus U-236 in a highly excited state. It is this nucleus, not the U-235 nucleus, that actually undergoes fission, splitting into two fragments. These fragments, between them, emit two neutrons, leaving Xe-140 and Sr-94 as fission fragments. Thus, the overal fission equation for this event is
![]()
The fragments Xe-140 and Sr-94 are both highly unstable and undergo beta decay (with the emission of an electron) until each reaches a stable end product.
The reason the nuclide U-236 is unstable is becuase the neutron/proton ratio is about 1.6 (144/92). The primary framents formed immediately after the fission reaction retain this same neutron/proton ratio. However, the stable nuclides in the middle-mass region have smaller neutron/proton ratios, in the rage of 1.3-1.4. That is the reason the fission fragments undergo beta decay.
Using the binding energy curve, we can estimate the energy released in this fission process. From this curve, we see that for heavy nuclides (mass about 240u), the mean biding energy per nucleon is about 7.6MeV. For middle-mass nuclides (mass about 120), it is about 8.5 MeV. This difference in total binding energy between a single large nucleus and two fragments (assumed to be equal) into which it may be split is then
![]()
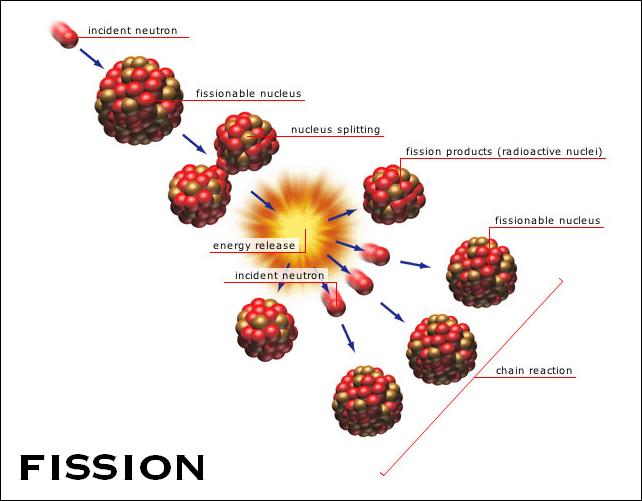
This is a relatively large amount of released energy per fission event. When a chain reaction occurs, many atoms and nuclei are involved so lots of energy is released. For a diagram of this particular chain reaction, see the diagram on the nuclear weapons page.
Fission in Nuclear Reactors
To make large-scale use of the energy released in fission, one fission event must trigger another, so that the process spreads thoughout the nuclear fuel as in a set of dominos. The fact that more neutrons are produced in fission than are consumed raises the possibility of a chain reaction. Such a reaction can be either rapid (as in an atomic bomb) or controlled (as in a reactor).
In a nuclear reactor, control rods made of cadmium or graphite or some other neutron-absorbing material are used to regulate the number of neutrons. The more exposed control rods, the less neutrons and vice versa. This also controls the multiplication factor k which is the ratio of the number of neutrons present at the beginning of a particular generation to the number present at the beginning of the next generation. For k=1, the operation of the reactor is said to be exactly critical, which is what we wish it to be for steady-power operation. Reactors are designed so that they are inherently supercritical (k>1); the multiplication factor is then adjusted to the critical operation by inserting the control rods.
An unavoidable feature of reactor operation is the accumulation of radioactive wastes, including both fission products and heavy "transuranic" nuclides such as plutonium and americium.
A Natural Fission Reactor
Some two billion years ago, in a uranium deposit now being mined in Gabon, West Africa, a natural fission reactor went into operation and ran for perhaps several hundred thousand years before shutting itself down. Investigation into this "natural" fission reactor concluded that it did in fact fission naturally. The investigation of neodymium was very convincing. In natural desposits of ore, Nd-142 occurs in more than 25% of all neodynium. However, in the natural fission reactor, there was no neodynium to be found. So, Enrico Fermi and his associates were wrong in assuming that they had put into operation the first fission reactor that had ever existed on this planet!